Medical Devices of Global Standards
Welford scores big with cutting-edge healthcare products

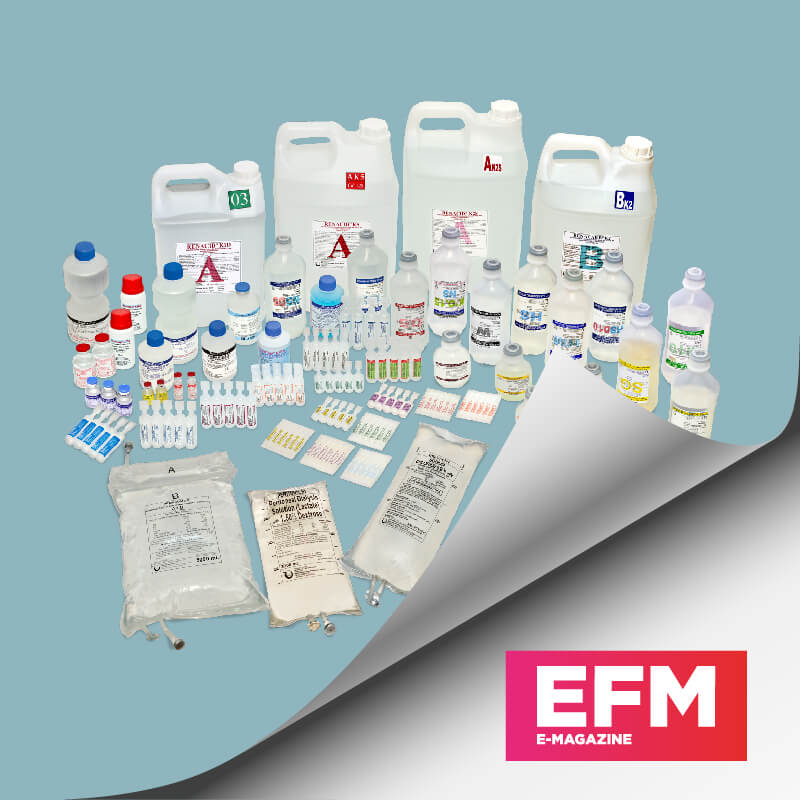
Infusion Therapy
From surgery to the path of recovery, patients need safe and effective healthcare products. From the beginning of diagnosis to the completion of treatment and care providing, healthcare professionals need reliable and quality healthcare devices.
Welford Manufacturing always strives to ensure that the well-being and comfort of the patients and healthcare professionals are not compromised.
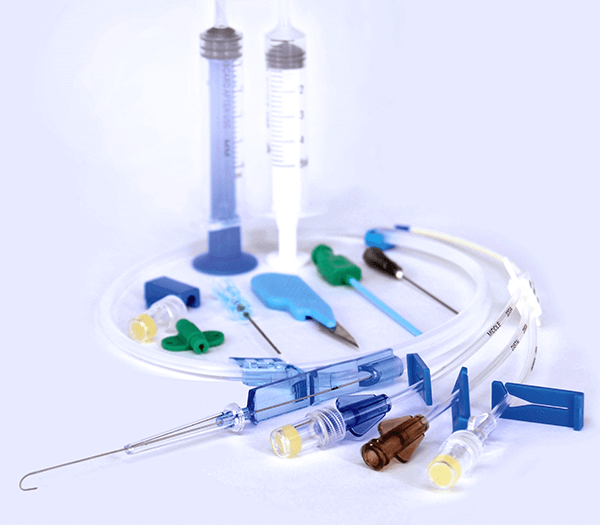
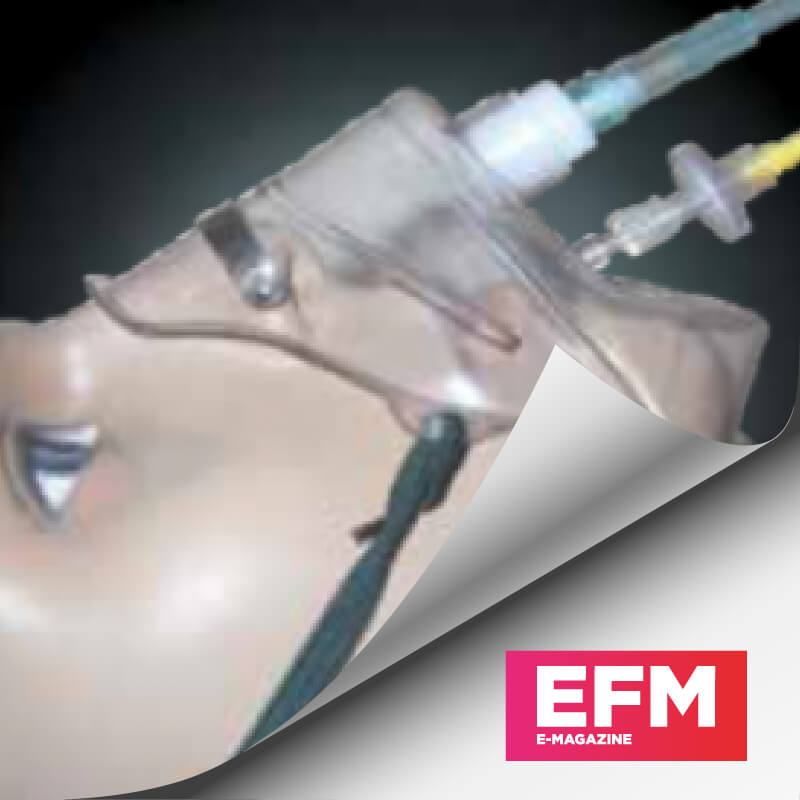
Central Venous Catheter Kit
Seasoned Company
Welford started as a manufacturer in the year of 1994, producing medical devices in the infusion lines and urology. The company primarily focuses on developing medical devices that have unique safety features by working closely with private and public institutions in studying and mitigating risks associated with clinical negligence, errors arising from clinical practices, and malfunctioning of devices.
Some of the company's products include infusion therapy – safety infusion sets, iv catheters, needleless extension sets, perfusion sets and others.
Today, Welford’s products are now being used in more than 35 countries worldwide, including the United Kingdom, Austria, Netherlands, Australia, Hong Kong, South Africa, Saudi Arabia, UAE, Malaysia, Greece, Malta, Bahrain, Oman, Philippines, Indonesia, Thailand, Vietnam, Myanmar, Kenya, Nepal, Sri Lanka, Cyprus, Syria, Pakistan, Bangladesh, Mauritius, Morocco, Jordan, Uganda, Ethiopia, Tanzania and others.
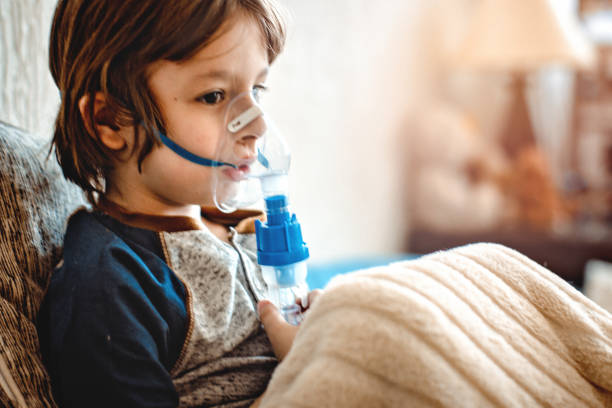
Respiratory Management
Exceptional R&D Capabilities
To meet the changing demands of the healthcare industry, Welford focuses on the continual process of product development.
Apart from our in-house research and development team, the outfit also works with the leading hospitals in the United Kingdom, universities, renowned research centres and companies in different regions to develop and design its medical devices.
From plastic materials characteristics to components design, from filtration media to production engineering, from device safety structure to prototyping, Welford equips itself with the best research & development capability to develop the most suitable devices for the medical industry.
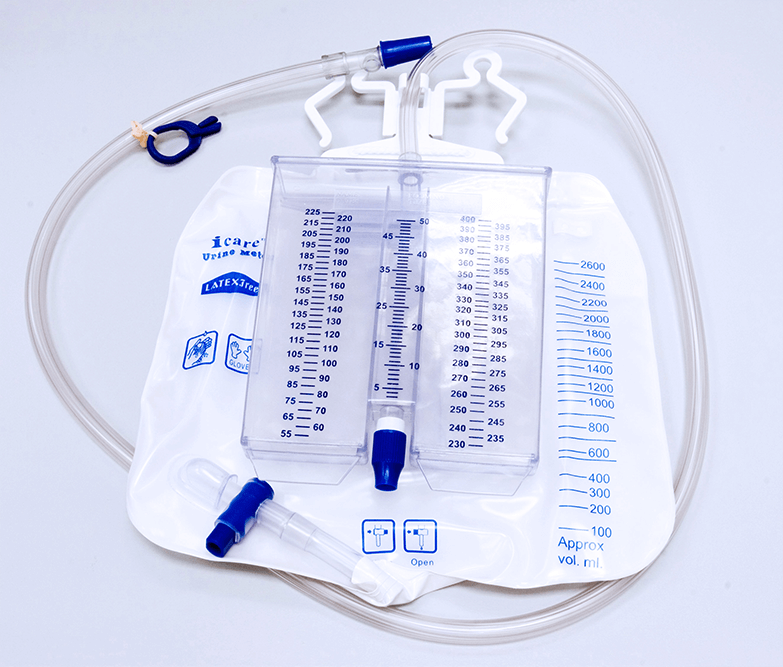
Urine Meter
Continuous Improvement
In ensuring safety and effectiveness of the company’s medical devices and in meeting and satisfying regulatory and customer's requirements and expectations, Welford establishes and maintains a quality management system. Throughout the implementation of the management system, all individuals in Welford are involved in the process of continuous improvement.
Its packaging process is challenged using the stringent US ASTM methods and is further qualified by the real-time and accelerated ageing tests. By this, the company is able to verify and guarantee the sterility and the shelf life of its devices, besides being recognised by its customers and regulatory bodies as a trustworthy and reliable supplier.
Adhering to Global Standards
Welford has also obtained the ISO 13485 certification from TUV SUD of Munich Germany. Its products carrying CE marking are certified in meeting the standards of the European Community Medical Design Directive MDD 93/42/EEC and hence are approved to be sold in the European markets.
To further ensure the safety of its devices, Welford places excellent emphasis on regulatory compliance. Products testing and regulatory documents are prepared and compiled to fulfil the standards of not only the European markets but also the competent authorities in other territories.

Contact Us

WELFORD MANUFACTURING (M) SDN BHD
25, Jalan BRP 9/1b, Kawasan Perindustrian Putra, 47000 Sungai Buloh, Selangor Darul Ehsan, Malaysia
Tel: +603-6150 6881 / +6012-4618 988
Fax: +603-6150 8877
Contact: Mr Wong Lun Cheng